Introduction
Neurodegenerative diseases (NDDs) affect a substantial and growing proportion of the aging population worldwide. Despite the increasing number of clinical trials aimed at exploring novel interventions for NDD, there is a substantial heterogeneity in the selection of appropriate instruments for outcome measurement. This study aimed to comprehensively investigate the outcome measurement instruments employed in clinical trials of NDD in past two decades. We collected the entry terms from Medical Subject Headings database and used as search keywords. An automatic pipeline was designed to retrieve clinical trials, download registration files, and extract meta information. The instruments employed in trials were manually recognized and normalized by domain experts. A total of 4619 trials posted from January 2001 to December 2022 were included for analysis, and 2494 unique instruments were identified. On average, 4.4 instruments were utilized per trial, with a minimum of 1 and maximum of 31. Only 15 instruments were shared across 6 common NDDs. 76.75% of the trials employed more than one instruments, with many instruments being employed in combinations with others. The top 10 frequently employed instruments for each disease were determined, and their temporal trends were continued to increase. we also explored the utilization patterns of sub-modules and different versions of instruments. This study provides a comprehensive investigation on implementation of instruments utilized in clinical trials of NDDs, through the utilization patterns discovery and temporal trends analysis. These findings have revealed employment heterogeneity of outcome measurement instruments, and provided valuable insights for better clinical practice of NDDs.
Figure 1. overview
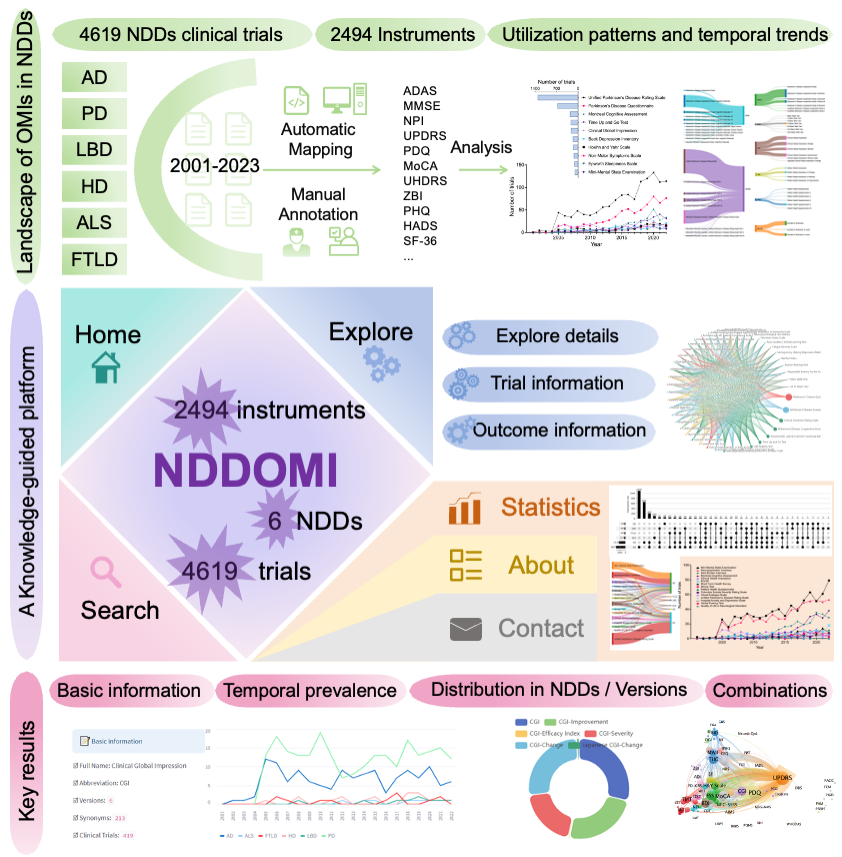
Search strategy
For each neurodegenerative disease, we collected entry terms from Medical Subject Headings (MeSH) database. The Mesh terms and Mesh entry terms were employed as keywords to retrieve clinical trials registration website up to January 2023. An overview of the data inclusion process is noted in Figure 1. We established several filtering criteria, including: (a) trails submitted before 2023; (b) trails related to the corresponding disease; and (c) trials with a description text of outcome measurement. A custom Python Script based pipeline was constructed to retrieve clinical trials, download registration files and extract information. The extracted information contains clinical trial identifier, title, date, condition or disease, outcome measurements. With the information, we removed trails that not matched the above criteria.
Instruments identification
To determine whether a trial applied any instruments, we manually annotated instrument entities within the description text of outcome measurements. The instruments annotation was performed using brat, a widely used web-based and user-friendly tool for text annotation in natural language processing research. Two annotators, the one is neurology clinician and the other is neuroscience researcher, collaborated to annotate the data. Initially, they surveyed the definitions and literatures of the outcome measurement instruments, and reviewed various expressions within clinical trials text. Then, the data was divided into two parts and labeled separately. Finally, they cross-checked each other's annotations to ensure consistency, and discussed contradictions until consensus was achieved. Based on the annotation results, we removed trials that did not applied any instruments in the outcome measurements.
Instruments normalization
Generally, an instrument comprises of multiple subscales, and maybe has various versions. To address this issue, we proposed a two-level instrument name normalization pipeline aimed at transforming original instrument expressions in the text into their corresponding normalized full names. Frist, we accounted for variability in writing styles, including punctuation marks, singular and plural numbers, among others, and we standardized various instruments expressions to formal expressions. Second, we considered the diversity of versions and modules, and standardized the formal expressions to higher level instrument. For example, the terms "Alzheimer’s Disease Assessment Scale – Cognitive Subscale" and "ADAS-cog" are different expressions, but actually refer to same instrument "Alzheimer’s Disease Assessment Scale - Cognitive Subscale". We defined it as a level 2 instrument and further mapped to the level 1 instrument "Alzheimer’s Disease Assessment Scale". Similarly, "Neuropsychiatric Inventory-Questionnaire" and “NPI-Q” are both normalized as instrument "Neuropsychiatric Inventory".
Contact us
If you have any questions, suggestions or feedback, please contact us:
E-mail: Bairong Shen (bairong.shen@scu.edu.cn); Hui Zong (zonghui0228@163.com)
Address: D5-A10, No.2222 Xinchuan Road, Chengdu 610041, Sichuan, China
Affiliation: Institutes for Systems Genetics, Frontiers Science Center for Disease-related Molecular Network, West China Hospital, Sichuan University